These products are useful for commercial purposes.
Some reduction reaction in drug manufacturing using. is wanted, how many grams of zinc are needed, in theory? The reaction takes place as follows . Gaseous hydrogen used in laboratory practice as a reducing agent. If neglected, an explosion can occur. Before collecting the hydrogen gas with the help of the apparatus, precautions must be taken in order to ensure that all the air inside the apparatus has been displaced. Hydrogen torches are used for welding purposes. These isotopes differ when the physical properties are considered because their atomic masses also differ., The following reaction can illustrate the chemical properties of hydrogen , Chemical reaction: 2H2 (g) + O2 (g) 2H2O. Hydrogen chloride is manufactured with the help of hydrogen gas. Reaction with metal ions and metal oxides some metal ions are reduced in an aqueous solution while the metal oxides are reduced into corresponding metals.
The knowledge of the space that we have today of our solar system and the heavenly bodies beyond would not have been possible without the use of hydrogen. Add the dilute hydrochloric acid into the flask containing granulated zinc through a thistle funnel.
 Reaction with dioxygen water is formed after this highly exothermic reaction. The uses are as follows . When hydrogenated in the presence of nickel as a catalyst, Vegetable oils produce edible fats. Zinc chloride is Answer the following pertaining to the Q5)In the industrial method of preparation of hydrogen by the Bosch process - give(a) Balanced equat Q6) State the following pertaining to the physical properties of hydrogen (a) Colour & odour (b) Sol Q7) Draw neat labelled diagrams for two experiments to prove that hydrogen is lighter than air. Granulated zinc is preferred over pure zinc because it provides a larger Ans. (a) It acts as a catalyst and increases the rate at which reaction is performed.
Reaction with dioxygen water is formed after this highly exothermic reaction. The uses are as follows . When hydrogenated in the presence of nickel as a catalyst, Vegetable oils produce edible fats. Zinc chloride is Answer the following pertaining to the Q5)In the industrial method of preparation of hydrogen by the Bosch process - give(a) Balanced equat Q6) State the following pertaining to the physical properties of hydrogen (a) Colour & odour (b) Sol Q7) Draw neat labelled diagrams for two experiments to prove that hydrogen is lighter than air. Granulated zinc is preferred over pure zinc because it provides a larger Ans. (a) It acts as a catalyst and increases the rate at which reaction is performed. 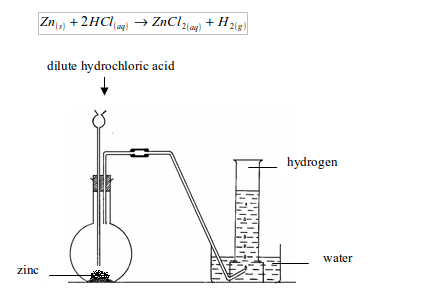 Ans. This gas is sparingly soluble in water. 1.A common laboratory preparation of hydrogen on a small scale Ammonia is formed when hydrogen is reacted with dinitrogen.
Ans. This gas is sparingly soluble in water. 1.A common laboratory preparation of hydrogen on a small scale Ammonia is formed when hydrogen is reacted with dinitrogen.
Part B- If the acid is available as
Part A- If 12.3 L of H2 at 765 torr and 20.0 C Reaction with halogens hydrogen halides are formed when reacted with halogens. We can denote the elements present in a compound in the form of symbols, along with their proportions, with the help of chemical formulae. Ans. Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. Name three compounds containing hydrogen in the co Q2) Starting from zinc how would you obtain hydrogen using(a)Steam (b) A dilute acid(c) An alkali( Q3) Hydrogen is obtained by electrolysis of acidified water. This helps in the acid reacting quickly with it. In the laboratory, hydrogen gas is produced by the reaction of granulated zinc with hydrochloric acid. Chemical reaction: H2+CO+RCH=CH2RCH2CH2CHO, Chemical reaction: 3H2 (g) +N2 (g) 2NH3. a volume of 869 cm3 at 794 torr and 25.0 C if it is heated to 60.0 It is best to use granulated zinc for the process of preparing hydrogen.
The uses of hydrogen are listed below , Get subscription and access unlimited live and recorded courses from Indias best educators. The build up of pressure ceases when all drops of acid left clinging to the solid have been used up. Ammonia is manufactured by using hydrogen. When gas is needed, the tap is turned on.
The process involves the electrolysis of brine solution. You are safe. The laboratory preparation of hydrogen gas usually involves the action of dilute sulphuric acid or dilute hydrochloric acid on zinc granules. It also is the first element of the periodic table. You must log in or register to reply here. Also, granulated zinc contains a small amount of copper, which acts as a catalyst.
This method is called the Haber process, and it is used to manufacture ammonia. Reactions with the organic compound In the presence of catalysts, hydrogen produces many hydrogenated products. Ans. Ammonia is formed when hydrogen is reacted with dinitrogen. There is no extra pressure to hold the acid in the top bulb, so it drops down to completely fill the bottom bulb and once more flood the solid.
It may not display this or other websites correctly. Helps in the manufacturing of hydrogen chloride. 7.61 M HCl, what is the minimum volume of this Electrolyzing warm aqueous barium hydroxide solution between nickel electrodes produces high purity (>99.95%) dihydrogen. This is because hydrogen gas reacts explosively with air. Kipp's apparatus is an elaborate piece of laboratory glassware used, until quite recently, for preparing and storing small volumes of certain gases, notably hydrogen. solution (in. Ammonia is formed when hydrogen is reacted with dinitrogen. solution (in milliliters) required to produce the amount of Q1) State how hydrogen occurs in the free state. Kipp's apparatus, also known as a Kipp generator, has now been superseded for the production of hydrogen by the use of acid and metal that convert to hydrogen gas.
The acid and zinc react with each other, producing hydrogen..
The purpose for the preparation of hydrogen gas, There are several uses of hydrogen gas for which hydrogen gas is produced. Hydrogen gas is a colourless gas which does not have any distinct odour. However, not all site features work fully without JS. Granulated zinc contains traces of impurities which act as catalyst and increase the rate of production of hydrogen. Learn how the catalyst reduces the activation energy and how to depict it in a potential energy diagram to speed up the reaction rate. The setup for the laboratory preparation of hydrogen gas is illustrated below. Welcome to the forum of professional participants of the drug market! Hydrogen is used to manufacture methanol and a number of other organic chemicals.. It makes us about 70% of the total mass of the universe., Hydrogen is non-poisonous, odourless, tasteless, and colourless at ordinary temperatures. 2022 Quality Tutorials Pvt Ltd All rights reserved, Allied Solutions
Granulated zinc is preferred over pure zinc because it provides a larger surface area. It also contains a small amount of copper, which acts as a catalyst in the process. It consists of one electron and one proton. 1.A common laboratory preparation of hydrogen on a small scale Hydrogenation in small scale with Pd/C catalyst, hydrogenation in small scale with pd/c catalyst, http://bbzzzsvqcrqtki6umym6itiixfhni37ybtt7mkbjyxn2pgllzxf2qgyd.onion/threads/hydrogen-gas-h2-lab. The hydrogen gas produced passes through a delivery tube and is collected by the downward displacement of water..
It is named after its inventor, the Dutch pharmacist Petrus Johannes Kipp (18081864). Class 8 A catalyst is a substance that increases the rate of a chemical reaction when it is introduced.
In the laboratory, hydrogen gas is produced by the reaction of granulated Ans. In manufacturing sodium hydroxide and chlorine, hydrogen is obtained as a byproduct. This method i Ans. Concept: Laboratory Preparation of Hydrogen, Chapter 7: Hydrogen - Objective Type Questions, Viraf J. Dalal Class 8 New Simplified Middle School Chemistry, Maharashtra Board Question Bank with Solutions (Official), Mumbai University Engineering Study Material, CBSE Previous Year Question Paper With Solution for Class 12 Arts, CBSE Previous Year Question Paper With Solution for Class 12 Commerce, CBSE Previous Year Question Paper With Solution for Class 12 Science, CBSE Previous Year Question Paper With Solution for Class 10, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Arts, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Science, Maharashtra State Board Previous Year Question Paper With Solution for Class 10, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Arts, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Commerce, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Science, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 10. Methanol and several other organic chemicals are produced by using hydrogen. When the gas tap is turned off, as the gas can no longer escape, the pressure again builds up, forcing the liquid back into the top bulb or reservoir. Get answers to the most common queries related to the NDA Examination Preparation.
Hydrogen is a combustible gas. H2 described in part (a)?
Part A- If 12.3 L of H2 at 765 torr and 20.0 C C and given a final volume of 1160 cm3?
Q4) In the laboratory preparation of hydrogen from zinc & dilute hydrochloric acid - state a reason for, (a) Addition of traces of copper [[II]] sulphate to the reaction medium, (b) Collecting the hydrogen by downward displacement of water and not air & collecting it after all the air in the apparatus is allowed to escape. There are several methods of preparing hydrogen gas in the laboratory and commercially. In the laboratory preparation of hydrogen from zinc and dilute hydrochloric acid the zinc used granulated zinc.
Zinc chloride is It is composed of three isotopes, and they are similar to each other in consideration of their chemical properties. Part B- If the acid is available as The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. This helps in the acid reacting quickly with it. Finally, the hydrogen gas can be collected by the downward displacement of water. This is so because the acid reacts with it quickly to form hydrogen since granulated zinc provides more surface area. What will be the final pressure of a sample of nitrogen with (c) Having the end of the thistle funnel dip below the level of the acid in the flask.
For example, you will not be able to watch our video tutorials. This method is called the Haber process, and it is used to manufacture ammonia. (b) Hydrogen is soluble in water and lighter than air. is wanted, how many grams of zinc are needed, in theory? Ans. During electrolysis, the reactions that take place are: Platinum electrodes are used for the electrolysis of acidified water to produce Hydrogen. Dilute hydrochloric acid is added into the flask containing granulated zinc through a thistle funnel.
the other product. As this mixture of CO and H2 is used for the synthesis of methanol and a number of hydrocarbons, it is also called synthesis gas or syngas. Hydrogen is usually produced by the reaction of zinc with dilute hydrochloric acid. Moreover, hydrogen is used as a fossil fuel. Learn about Hydrogen gas and the preparation of this gas in the laboratory in this study material on the preparation of hydrogen gas. The gas pressure in the center bulb is released. 2003-2022 Chegg Inc. All rights reserved. Ans.
We review their content and use your feedback to keep the quality high.
Thus granulated zinc is preferred over pure zinc to produce hydrogen in the laboratory. So, it must be ensured that air inside all the apparatus being used has been removed. Amongst all the elements around us in nature, hydrogen has the simplest atomic structure. the other product. The different ways through which hydrogen is produced commercially is given below , Over anode: 2Cl(aq) Cl2(g) + 2e, Over cathode: 2H2O (l) + 2e H2(g) + 2OH(aq), The overall reaction: 2Na+ (aq) + 2Cl(aq) + 2H2O(l)Cl2(g) + H2(g) + 2Na+ (aq) + 2OH(aq), Reaction: CH4 (g) + H2O CO (g) + 3H2. uses the reaction of zinc with hydrochloric acid. An experimental procedure for the laboratory preparation of hydrogen gas is provided below. Granulated zinc is ideal for the preparation of hydrogen gas in chemical laboratories because it usually contains a small amount of copper, which has the ability to act as a catalyst to the associated chemical reaction and, therefore, increase the rate of the chemical reaction without actually participating in it.
The reaction takes place as follows . Here you will get all the necessary information about organizing a laboratory of any size, from a small kitchen at home to an industrial facility.And if you have your own production, here you will find all the relevant information to improve efficiency and safety.In the sections of the forum you will find: JavaScript is disabled. Procedure for preparing hydrogen with the reaction of zinc and dilute hydrochloric acid, Procedure for preparing hydrogen with the reaction of zinc with aqueous alkali, Zinc is reacted with boiling aqueous alkali and forms hydrogen, Precautions to be taken in the laboratory while preparing hydrogen.
There are various methods of preparing hydrogen gas. The solubility of this gas in water is not affected too much by any changes in temperature. Through this article, readers will get deep insights into the concepts of what is atmosphere, the different layers of atmosphere and various reactions taking place in different layers of the atmosphere. Dihydrogen is the most abundant element in the universe. Unacademy is Indias largest online learning platform. Get all the important information related to the NDA Exam including the process of application, syllabus, eligibility criteria, exam centers etc. This method is called the Haber process, and it is used to manufacture ammonia. Granulated zinc is preferred over pure zinc because it provides a larger surface area. At high temperatures, the reaction of steam on hydrocarbons or coke in the presence of a catalyst produces hydrogen. Descriptions of the pharmacological action of substances. Also, granulated zinc contains a small amount of copper, which acts as a catalyst.
Ans. 7.61 M HCl, what is the minimum volume of this An electrode is a good source of conducting electricity as it is a solid conductor, in other words we can use Anode and Cathode.
Is magnesium hydride MgH2 an ionic compound class 12 chemistry JEE_Main, Write the equations for the preparation of 1iodobutane class 12 chemistry JEE_Main, The degree of hydrolysis for a salt of strong acid class 11 chemistry JEE_Main, The ratio of KpKcfor the reaction COg + dfrac12O2g class 11 chemistry JEE_Main, The reaction COg + 3H2g leftrightarrow CH4g + H2O is class 12 chemistry JEE_Main, Poly beta hydroxybutyrateco beta hydroxy valerate PHBV class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Your browser is not able to display this video. However, it exists as a diatomic molecule (H2) in its elemental form and is referred to as dihydrogen. Ammonia is manufactured using hydrogen, which further helps in manufacturing nitric acid. A catalyst is a material that is not consumed by a chemical process but reduces the activation energy of the reaction. There are many applications of hydrogen gas in the manufacturing of certain chemicals that are used vastly. uses the reaction of zinc with hydrochloric acid.
Some uses of hydrogen gas are listed below. Reaction with dinitrogen ammonia is formed when hydrogen is reacted with dinitrogen. Experts are tested by Chegg as specialists in their subject area. In the laboratory, hydrogen gas is produced by the reaction of granulated zinc with hydrochloric acid. The uses of hydrogen are listed below . 1) The reaction is: A) you nee to calculate the moles of hydrogen, using the ideal gas law: ----->R=62.363L.torr mol-1K.
Hydrogen is colourless, tasteless, and odourless. 2. However, we do not notice it in our daily lives. The hydrogen gas produced passes through a delivery tube and is collected by the downward displacement of water. You are using an out of date browser. Chemistry. Example Vegetable oils, when hydrogenated in the presence of nickel as a catalyst, produce edible fats. The mixture of CO and H2 is called water gas. Hydrogen helps in generating electric energy through fuel cells.